The GalD Assay provides a robust means to measure serum galactose-deficient IgA1 (Gd-IgA1) levels across cohorts and centers, and in serial samples. The assay is based on a lectin binding to any of the accessible galactose-deficient sites of multiple disease-causing forms of Gd-IgA1. Lectin-based measurements of serum Gd-IgA1 served as the basis for the 4-Hit hypothesis of IgAN and revealed multiple disease-associated forms of Gd-IgA1. The GalD Assay provides the most comprehensive assessment of Gd-IgA1 relevant to IgAN pathogenesis and enables any group to integrate quantification of this proven serum biomarker into their IgAN studies.
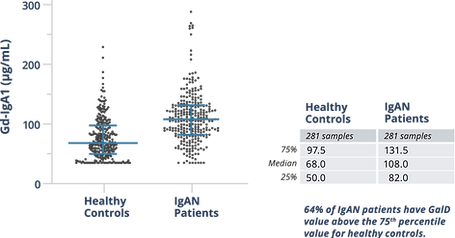
Analysis of serum samples from 3 distinct cohorts with the GalD Assay showed differences between the median serum Gd-IgA1 levels of healthy controls and patients with IgAN. More than half of IgAN patients had GalD values >75th percentile of healthy controls.

Potential Uses of


Compare IgAN cohorts from various studies:
-
Across academic cohorts
-
Across clinical trials
-
Enable true longitudinal analysis
Risk-stratify patients to:
-
Establish risk thresholds of Gd-IgA1 levels
-
Identify Gd-IgA1 target levels
-
Prioritize for biopsy
Monitoring IgAN patients individually over time:
-
Selection of disease-modifying treatment
-
Monitoring patient response to treatment and after interrupting treatment
-
Identifying patients at high risk of recurrent disease




